-
Die Universität
- Herzlich willkommen
- Das sind wir
- Medien & PR
-
Studium
- Allgemein
- Studienangebot
- Campusleben
-
Forschung
- Profil
- Infrastruktur
- Kooperationen
- Services
-
Karriere
- Arbeitgeberin Med Uni Graz
- Potenziale
- Arbeitsumfeld
- Offene Stellen
-
Diagnostik
- Patient*innen
- Zuweiser*innen
-
Gesundheitsthemen
- Gesundheitsinfrastruktur
Myeloid Cells & Leukemia
In der Forschungseinheit „Myeloid Cells & Leukemia“ beschäftigen wir uns mit Aspekten maligner Hämatopoese, im speziellen der akuten myeloischen Leukämie (AML). Diese heterogene Erkrankung, die in jedem Lebensalter auftreten kann, zeigt in hohem Maße Resistenz gegenüber konventionellen Behandlungen. Unser Ziel ist es, grundlegende molekulare Mechanismen der Krankheitsentstehung und Resistenzentwicklung zu verstehen, um so zu innovativen Strategien beizutragen.
Ansprechpartner
Schwerpunkte
Grundlagenforschung
AML mit Aberrationen im TP53 Gen sind durch charakteristische genomische Veränderungen und ein überaus schlechtes Therapieansprechen charakterisiert. In Grundlagenforschungsprojekten gehen wir der Frage nach, wie TP53 Aberrationen als frühe leukämogene Ereignisse hämatopoetische Stamm- und Vorläuferzellen transformieren. Dabei bedienen wir uns der CRISPR/Cas9 Technologie wie auch in vivo Xenograft Modellen. Überdies untersuchen wir an isogenen Leukämie-Zelllinien, welche Mechanismen Resistenz gegenüber herkömmlichen Chemotherapeutika vermitteln und wie diese aberranten Signaltransduktionswege spezifisch inhibiert werden könnnen.
Die Forschungsarbeiten werden vom Österr. Wissenschaftsfonds FWF und der Leukämiehilfe Steiermark unterstützt.
Leukämie Biobank
Primäre Proben von Patient*innen mit Leukämie stellen ein wertvolles Biomaterial zur Erforschung von krankheitsspezifischen Veränderungen wie auch Prüfung der Wirksamkeit neuer Arzneimittel dar. In der Leukämie Biobank, die 1995 gegründet wurde, werden Proben mit dem Schwerpunkt AML vital tiefgefroren. Jede einzelne Probe weist eine detaillierte genetische Charakterisiserung auf, überdies stehen klinische Angaben von Patient*innen und deren Therapien zur Verfügung.
Neben dem Nutzen für die Forschungsaktivitäten der Abteilung dienen die Proben auch Kooperationsprojekten mit akademischen Zentren weltweit wie auch der pharmazeutischen Industrie.
Klinische Studien
Klinische Studien sind ein unabdingbares Mittel, neue Arzneimittel und therapeutische Strategien unter streng kontrollierten Bedingungen am Menschen zu prüfen. Die Forschungsgruppe „Myeloid Cells & Leukemia“ ist Partner sowohl von akademischen Studien als Mitglied der Deutsch-Österreichischen „Acute Myeloid Leukemia Study Group“ (AMLSG) als auch jenen der pharmazeutischen Industrie.
Grundlagenforschung
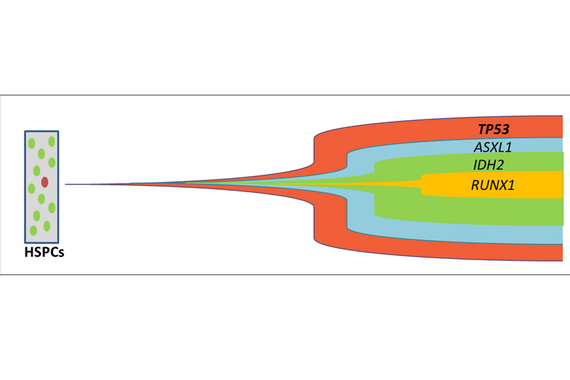
TP53 Mutationen sind frühe Ereignisse der akuten myeloischen Leukämogenese, die präleukämische Stammzellen betreffen. Adaptiert nach Lal R et al. Blood 2017.
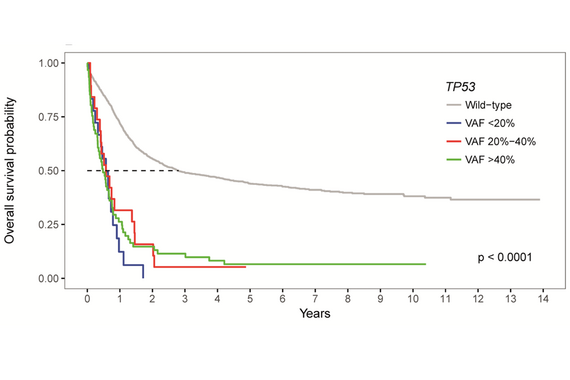
Klonale wie auch subklone TP53 Mutationen stellen adverse prognostische Parameter bei Patient*innen mit AML dar. Adaptiert nach Prochazka KT et al. Haematologica 2019.
Team
- Ilona Brandstätter
- Sybille Hofer
- Karin Lind
- Sayantanee Dutta
- Florian Zöscher