-
Die Universität
- Herzlich willkommen
- Das sind wir
- Medien & PR
-
Studium
- Allgemein
- Studienangebot
- Campusleben
-
Forschung
- Profil
- Infrastruktur
- Kooperationen
- Services
-
Karriere
- Arbeitgeberin Med Uni Graz
- Potenziale
- Arbeitsumfeld
- Offene Stellen
-
Diagnostik
- Patient*innen
- Zuweiser*innen
-
Gesundheitsthemen
- Gesundheitsinfrastruktur
Case of the Month
December 2021
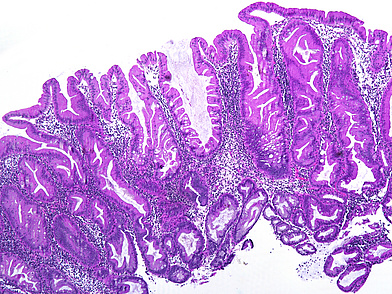
Ascending colon polyp in a 79-year-old male.
Diagnosis:
Unclassified serrated adenoma.
Comment:
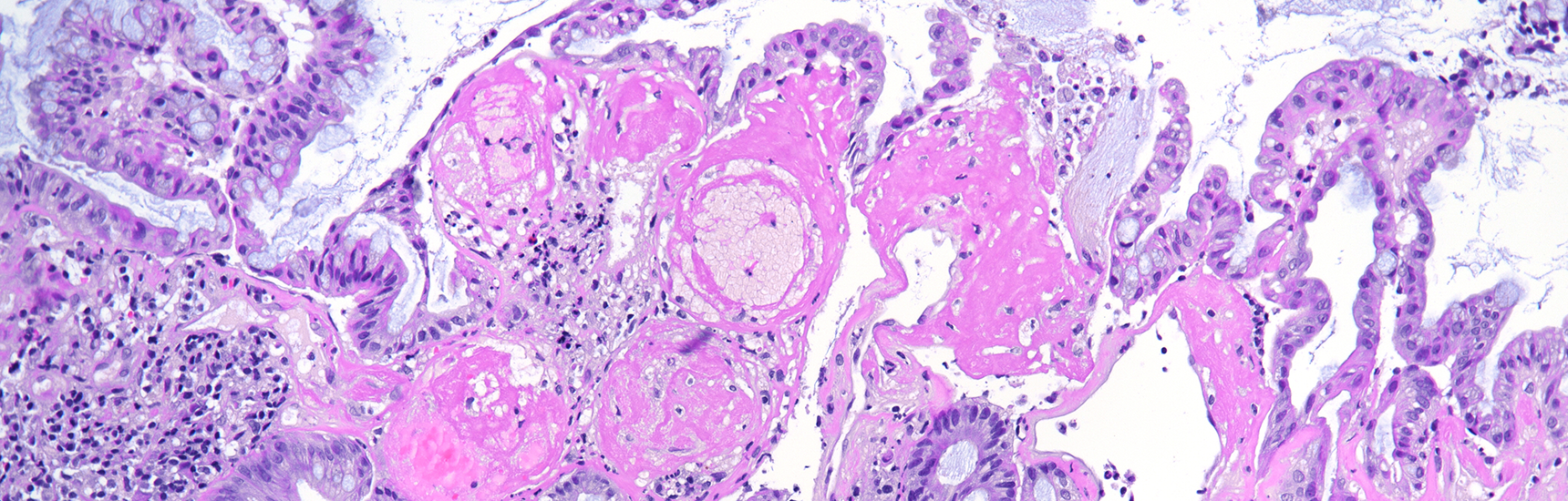
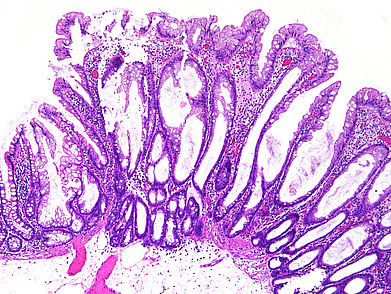
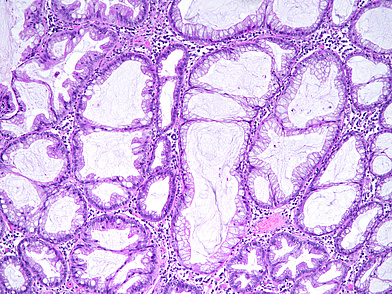
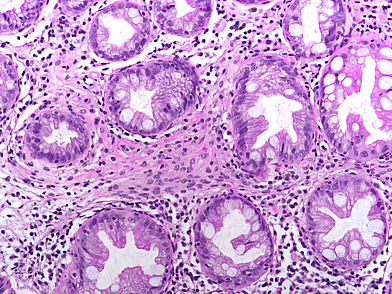
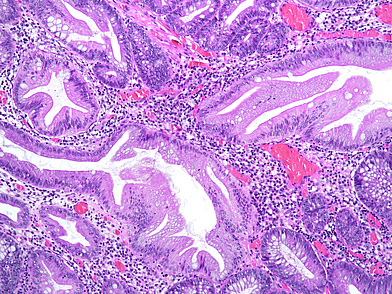
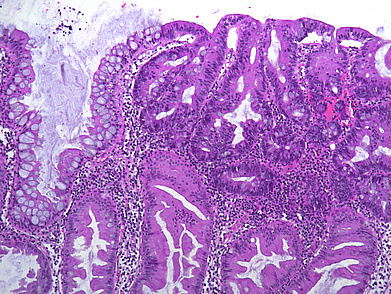
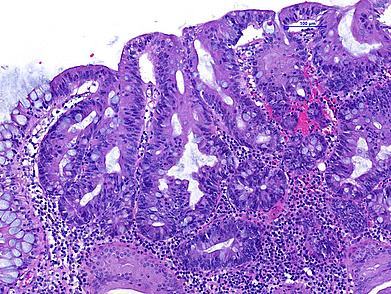
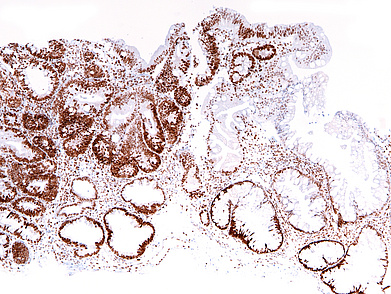
The ascending colon polyp is a serrated polyp characterized by a combination of two morphological patterns which are sessile serrated lesion (SSL) and traditional serrated adenoma (TSA). The former is formed by architecturally distorted serrated crypts with deep serrations, basal crypt dilatation and lateral growth along the muscularis mucosae (Panels A-B). Some foci of stromal proliferation resembling perineural cells are also seen within this component (Panel C). The TSA component has typical slit-like serrations, lined by tall columnar cells with intensely eosinophilic cytoplasm and pencillate nuclei (Panels D-E). Abrupt transition to dysplastic mucosa and glands are present, where the glands display more complex and crowded architecture with little intervening lamina propria (Panel F). The dysplastic cells show features of adenomatous dysplasia characterized by columnar cells with enlarged hyperchromatic nuclei, pseudostratification and reduced goblet cells (Panel G). In some areas, the cells display high grade cytology with more rounded nuclei and loss of polarity. MLH1 immunostaining was preserved in both the non-dysplastic and dysplastic glands with higher intense staining seen in the latter (Panel H).
The variable morphological features that is present in this polyp makes it difficult to delineate whether this polyp represents a SSL with dysplasia (SSLD) or TSA with overt (adenomatous) dysplasia. Retained MLH1 staining pattern was also not helpful in this case as it is known that both adenomatous and serrated- type dysplasia in SSL will show preserved MLH1 staining. Therefore, when dealing with such complexity in a polyp, it is best to classify such polyp as unclassified serrated adenoma (USA).
This category has been recently added in the 2019 WHO 5th edition, classification of serrated colorectal lesions which includes lesions that are difficult to classify as SSLD or TSA and serrated tubulovillous adenoma. Serrated tubulovillous adenoma has been described by Bettington et al, as a distinct variant of conventional tubulovillous adenoma characterised by the unique pattern of serration (festoon/undulating pattern) occupying more than 50% of the polyp. Other histological features includes: >25% villous component, TSA cytology and slit-like serration <10% of the polyp, more proximal location, larger size and having more CpG island methylation and KRAS mutations. Serrated tubulovillous adenoma can be easily misdiagnosed as TSA especially when considering that TSA can also coexist with conventional tubulovillous adenoma. Some histological features that can be helpful in differentiating TSA from serrated tubulovillous adenoma are the lack of intense eosinophilic cytoplasm and characteristic slit-like serration in the latter. We believe, however, that conventional tubulovillous and villous adenomas showing only minor foci of TSA morphology should better be classified as conventional (tubulovillous or villous) adenomas and not as an “unclassifiable lesion”.
The other controversial points in serrated polyp diagnosis is the significance of traditional serrated adenoma-type cells in various colonic lesions. Some authors calls this enteric metaplasia, which have characteristics of senescent cells rather than dysplastic cells while others consider that the presence of this cell type in a serrated polyp represents a form of serrated dysplasia. The different opinions can lead to the confusion in the differential diagnosis between TSA and SSLD. Rish K.Pai et all recommends that the term ‘serrated dysplasia’ should only be used for the “high-grade” dysplastic changes.
Inflammatory pseudopolyps and mucosal prolapse of the large intestine can also exhibit the same eosinophilic cell changes seen in TSA and display some serration mimicking a SSL or TSA. The differential diagnosis between these entities can occasionally be challenging. Inflammatory pseudopolyps and mucosal prolapse of the large intestine have more marked inflammation in the lamina propria and crypts show more obvious architectural distortion.
Should we grade the degree of dysplasia (low grade versus high grade) in the diagnosis of our lesion? This is a matter of debate that cannot be solved at the moment. The following issues need to be considered: (i) SSLD is by definition not graded, (ii) overt dysplasia in TSA is usually high grade and therefore also not graded (not touching the point whether TSA lacking overt dysplasia is low grade dysplastic in itself or should not be regarded as a dysplastic lesion). Therefore, we presented our impression of the grade of dysplasia only in the histological description.
While setting some approach and diagnostic criteria can resolve the diagnostic dilemma for most lesions, some polyps still remain difficult for categorization. As mentioned, a diagnosis of unclassified serrated adenoma (USA) can be appropriate in this setting. However, we should use this term sparingly and try as much as possible to categorize a lesion with serrated morphology as this would help in proper management and surveillance of the patient.
For further reading:
- Runjan Chetty, Traditional serrated adenoma (TSA): morphological questions, queries and quandaries. J Clin Pathol 2016; 69: 6-11
- Mark Bettington Neal Walker, Christophe Rosty, Ian Brown, Andrew Clouston, Diane McKeone, Sally-Ann Pearson, Kerenaftali Klein, Barbara Leggett & Vicki Whitehall, Serrated tubulovillous adenoma of the large intestine. Histopathology 2016; 68: 578–587
- Cheng Liu, Neal I Walker, Barbara A Leggett, Vicki LJ Whitehall, Mark L Bettington and Christophe Rosty, Sessile serrated adenomas with dysplasia: morphological patterns and correlations with MLH1 immunohistochemistry. Modern Pathology 2017; 30: 1728–1738
- Rish K. Pai, Mark Bettington, Amitabh Srivastava, Christophe Rosty, An update on the morphology and molecular pathology of serrated colorectal polyps and associated carcinomas. Modern Pathology 2019; 32: 1390–1415
Presented by:
Presented by Dr. Maalini Krishnasamy, Malaysia, and Dr. Cord Langner, Austria.