-
Die Universität
- Herzlich willkommen
- Das sind wir
- Medien & PR
-
Studium
- Allgemein
- Studienangebot
- Campusleben
-
Forschung
- Profil
- Infrastruktur
- Kooperationen
- Services
-
Karriere
- Arbeitgeberin Med Uni Graz
- Potenziale
- Arbeitsumfeld
- Offene Stellen
-
Diagnostik
- Patient*innen
- Zuweiser*innen
-
Gesundheitsthemen
- Gesundheitsinfrastruktur
Case of the Month
March 2025
Biopsy of stomach and duodenum from a 74-year-old male patient with known esophageal squamous cell carcinoma.
Diagnosis
Paclitaxel therapy-related gastroduodenitis.
Comment
A 74-year-old male patient with squamous cell carcinoma of the esophagus, who has been receiving neoadjuvant combined chemoradiotherapy according to the CROSS trial regimen for the past four weeks, was admitted for an upper gastrointestinal endoscopy due to the progression of dysphagia.
Endoscopy reveals diffuse tumor infiltration with ulceration involving the lower, mid and upper thirds of esophagus. Gastric and duodenal mucosa show no specific findings.
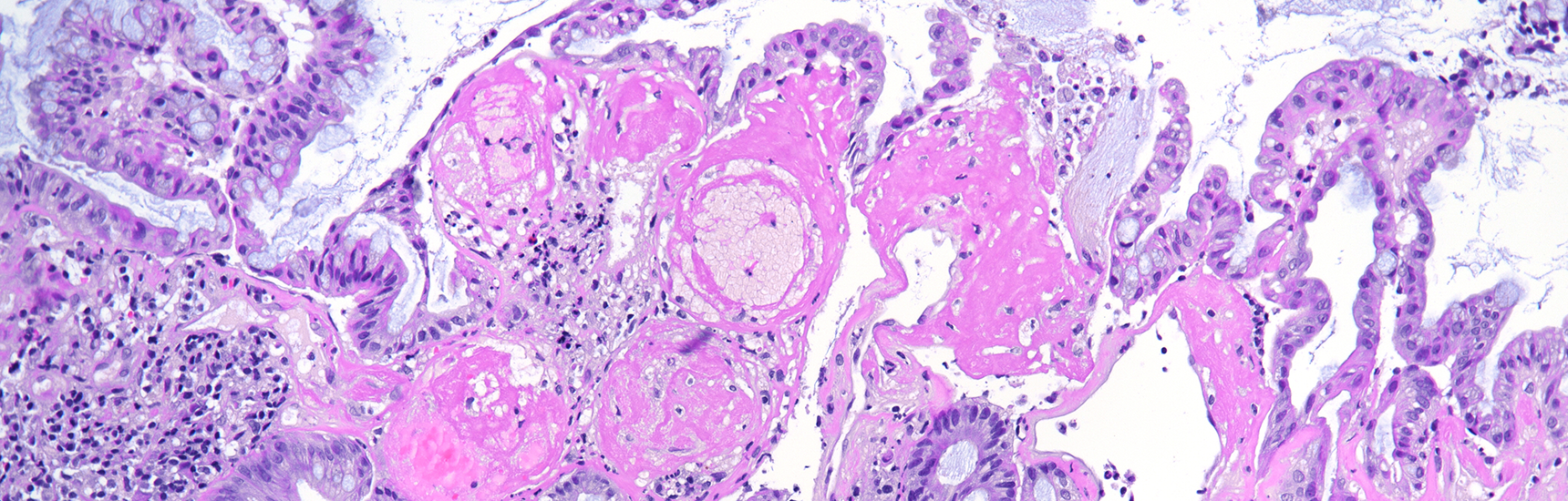
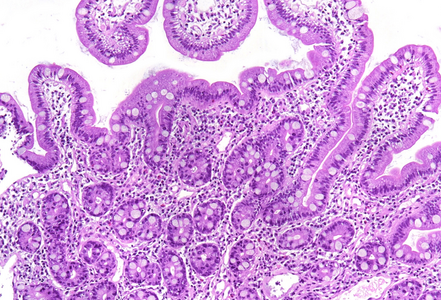
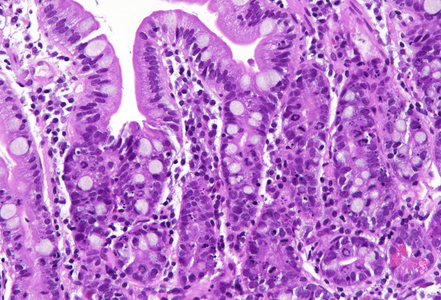
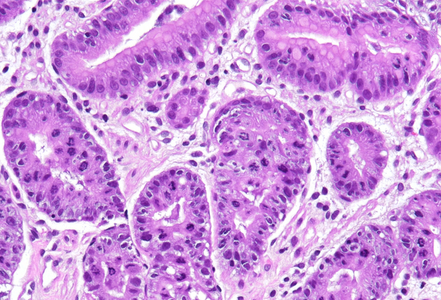
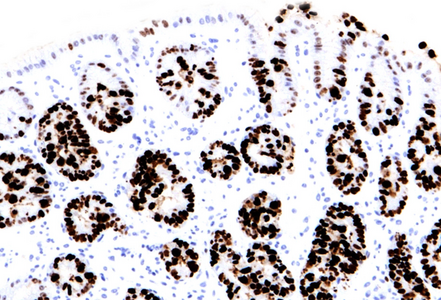
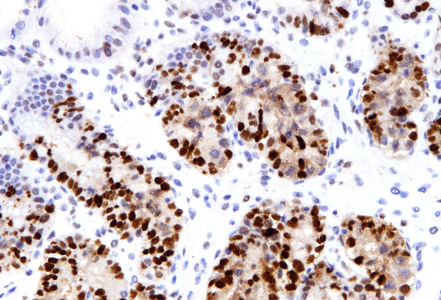
Histologically, duodenal mucosa shows preserved architecture and normal cell content within the lamina propria (Panel A). On high magnification we see a striking increase in mitotic figures, with multiple atypical ring mitoses, as well as apoptotic bodies in the crypt epithelium. Some nuclear enlargement with loss of nuclear polarity in the crypt epithelium is also observed (Panel B). Similar changes, with multiple ring mitoses in glands epithelium, increased apoptosis and mild active inflammation, is also observed in the gastric antral mucosa (Panel C). The cells with ring mitoses are immunoreactive for MIB-1/Ki-67 (Panel D) and p53 (Panel E). The histology and the results of immunohistochemical reactions correspond to “mitotic arrest” that is known to be caused by certain drugs, including colchicine, docetaxel and paclitaxel. The CROSS trial chemoradiotherapy regimen, received by the patient, includes weekly cycles of carboplatin and paxlitaxel. Hereby, the diagnosis of paclitaxel therapy-related gastroduodenitis is made.
The described above histological evidence of altered mitotic cell division caused by paclitaxel, unlike colchicine, reportedly has no significant correlation to the severity of paclitaxel toxicity symptoms. Also, rare ring mitoses may be seen in GI biopsies from untreated patients without taxane or colchicine exposure.
Of note, histologic changes as described under taxane drug administration (increased mitoses with atypical mitotic figures, p53 overexpression) should not be misinterpreted as dysplasia. In contrast to dysplastic epithelial changes, those associated with taxanes are restricted to the proliferative compartment and thus biopsies display surface maturation; the mitotic activity and loss of polarity stop abruptly beneath the epithelial surface, as illustrated in our H&E stained images.
Carboplatin, another drug received by the patient, reportedly cause acute inflammation in gastrointestinal mucosa with cytopathic epithelial features, such as increased cytoplasmic eosinophilia with marked nuclear atypia as well as increased apoptosis (compare the ENGIP Case of the Month in March 2015). Mitotic arrest is usually not observed under carboplatin treatment.
For further reading
- Daniels JA, Gibson MK, Xu L, Sun S, Canto MI, Heath E, Wang J, Brock M, Montgomery E. Gastrointestinal tract epithelial changes associated with taxanes: marker of drug toxicity versus effect. Am J Surg Pathol. 2008 Mar;32(3):473-7. doi: 10.1097/PAS.0b013e3181582331. PMID: 18300801.
- Langner C, Ott A. Possible pitfall in diagnosis: mitotic arrest of gastric epithelium after docetaxel therapy for hormone-refractory prostatic cancer. Histopathology. 2007 Jul;51(1):111-3. doi: 10.1111/j.1365-2559.2007.02672.x. Epub 2007 Apr 26. PMID: 17466032.
- Ma C, Montgomery E, Lam-Himlin D. Docetaxel therapy induced diffuse atypia and mitotic arrest mimicking dysplasia – a diagnostic pitfall. Diagnostic Histopathology, Volume 20, Issue 1, 46 – 47. DOI: 10.1016/j.mpdhp.2013.12.003.
- Noordman BJ, Verdam MGE, et al; CROSS Study Group. Impact of neoadjuvant chemoradiotherapy on health-related quality of life in long-term survivors of esophageal or junctional cancer: results from the randomized CROSS trial. Ann Oncol. 2018 Feb 1;29(2):445-451. doi: 10.1093/annonc/mdx726. PMID: 29126244.
- Abu-Sbeih H, et al. Gastrointestinal toxic effects in patients with cancer receiving platinum-based therapy. J Cancer. 2020 Mar 4;11(11):3144-3150. doi: 10.7150/jca.37777. PMID: 32231718; PMCID: PMC7097936.
Presented by
Dr. Hanna Lapsar, Kyiv, Ukraine, and Dr. Cord Langner, Graz, Austria.