-
Die Universität
- Herzlich willkommen
- Das sind wir
- Medien & PR
-
Studium
- Allgemein
- Studienangebot
- Campusleben
-
Forschung
- Profil
- Infrastruktur
- Kooperationen
- Services
-
Karriere
- Arbeitgeberin Med Uni Graz
- Potenziale
- Arbeitsumfeld
- Offene Stellen
-
Diagnostik
- Patient*innen
- Zuweiser*innen
-
Gesundheitsthemen
- Gesundheitsinfrastruktur
Case of the Month
November 2025
Polyp in the sigmoid colon of a 31-year-old woman.
Diagnosis
Peutz–Jeghers polyp (with beginning pseudoinvasion; syn. misplaced non-neoplastic mucosa).
Comment
A 31-year-old female patient with history of recurrent episodes of hematochezia underwent colonoscopy, which revealed a 25 mm stalked polyp, classified as NICE type 3. The polyp was completely removed via snare polypectomy. This was the single lesion in the large bowel, and upper gastrointestinal endoscopy showed likewise no other lesions or abnormalities.
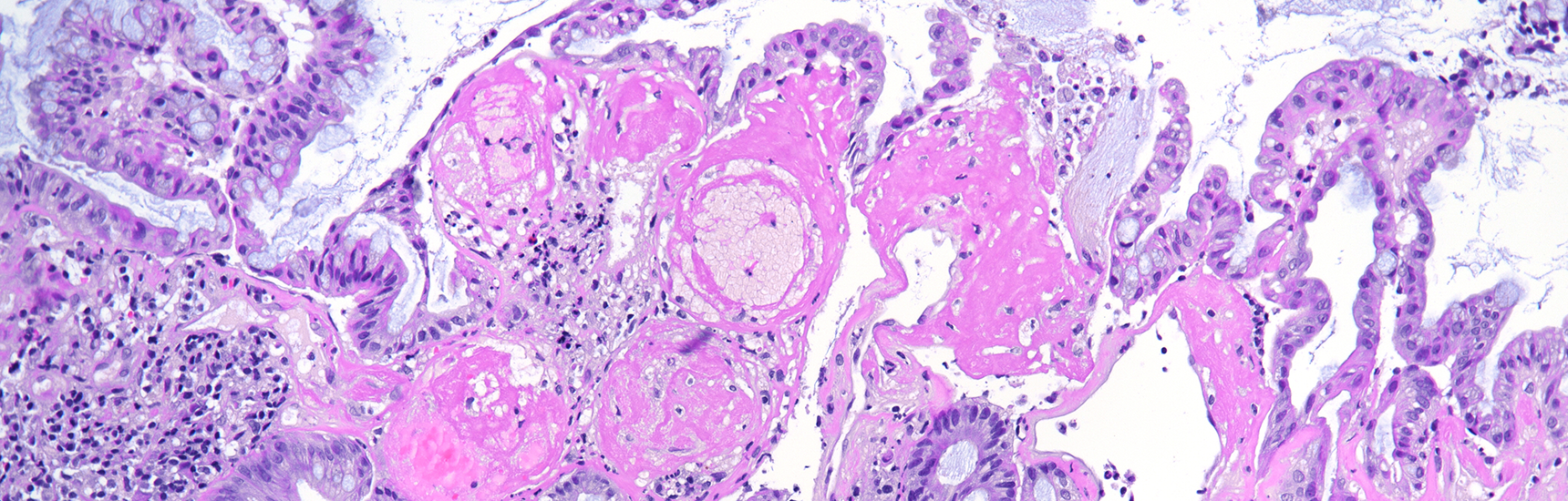
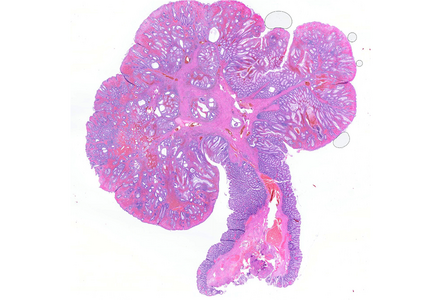
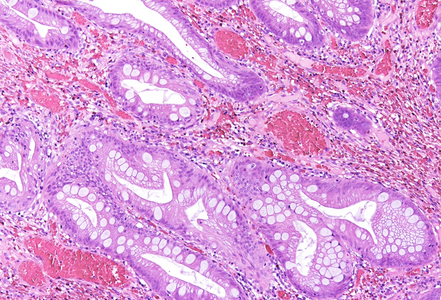
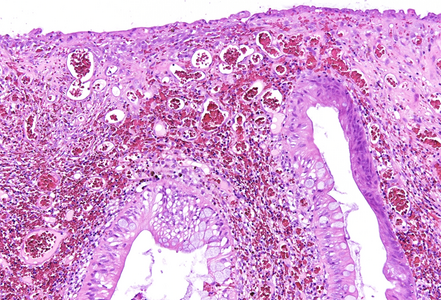
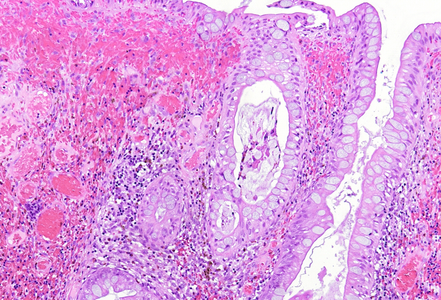
Histologically, the lesion exhibited a complex lobulated architecture (Panel A), with central cores of ramifying bands of smooth muscle extending from the muscularis mucosae into the lamina propria, configuring an arborizing pattern (Panel B). The epithelium exhibited neither cytologic nor architectural atypia and was regarded as reactive (non-dysplastic), with some glands showing dilation and distortion (Panel C). Recovering epithelium was observed on the surface, together with granulation tissue (Panel D).
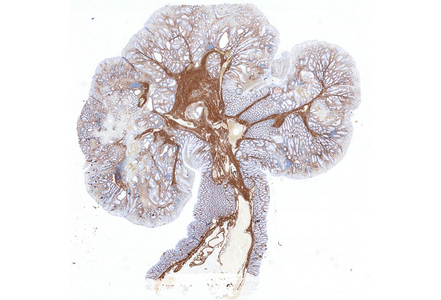
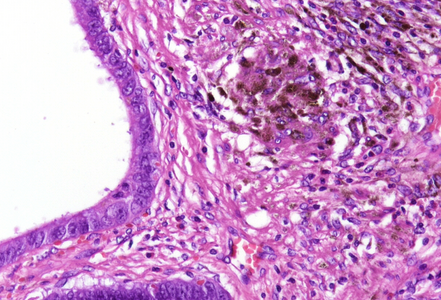
Notably, the lesion demonstrated beginning pseudo-invasion, defined by the presence of misplaced benign glandular elements extending into the upper part of the stalk. There was mild inflammation of the lamina propria, abundant hemorrhage and hemosiderin-laden macrophages were also observed (Panels E-F). Immunohistochemistry for desmin and smooth muscle actin (SMA) was performed to highlight the smooth muscle fibers (compare Panel B) and emphasize the typical lobulated and arborizing architecture of this type of polyp.
A Peutz-Jeghers polyp (PJP) is a rare hamartomatous lesion that may occur sporadically or, more commonly, in association with Peutz-Jeghers syndrome, a hereditary disorder characterized by mucocutaneous pigmentation (most often involving the oral mucosa), hamartomatous polyposis, and an increased lifetime risk of various malignancies. Over 90% of individuals with this syndrome harbor a germline mutation in the tumor suppressor gene STK11.
PJPs can occur throughout the gastrointestinal tract but are most prevalent in the small bowel (particularly the jejunum and ileum), followed by the colon and stomach. Histologically, they are characterized by villous or lobulated architecture and arborizing bundles of compact smooth muscle dividing relatively normal lobules of glandular mucosa.
Differential diagnoses include mucosal prolapse polyps, which exhibit disorganized smooth muscle wrapping around individual crypts and are more common in the rectosigmoid colon, and juvenile polyps, which display a prominently inflamed and edematous stroma, marked cystic dilation of epithelial glands, and absence of smooth muscle fibers surrounding the lobules.
A well-recognized diagnostic pitfall in the histopathologic evaluation of PJPs is the phenomenon of pseudo-invasion, observed in approximately 10% of cases and preferably in the small bowel. This feature can closely mimic invasive adenocarcinoma. Pseudo-invasion refers to the misplacement of benign epithelial glands into the submucosa, muscularis propria, or even the serosa, often accompanied by stromal reaction and hemorrhage. This phenomenon is most commonly seen in pedunculated polyps and is believed to result from mechanical forces, including peristalsis, prolapse, and traction on the polyp stalk.
Histologically, pseudo-invasion is defined by benign-appearing glandular epithelium, typically lined by mucosa identical to the surface component, without significant cytologic atypia. These glands are usually accompanied by lamina propria-like stroma, rather than a desmoplastic reaction, and there is typically no evidence of destructive stromal invasion - features that help differentiate it from true carcinoma. The presence of mucus lakes is also characteristic.
In ambiguous cases, immunohistochemical markers are generally of limited value, and diagnosis should be based on recognition of the overall histologic context, the absence of cytologic atypia and desmoplasia, and correlation with clinical and endoscopic findings.
For further reading
- Petersen VC, Sheehan AL, Bryan RL, Armstrong CP, Shepherd NA. Misplacement of dysplastic epithelium in Peutz-Jeghers polyps: the ultimate diagnostic pitfall? Am J Surg Pathol. 2000 Jan;24(1):34-9
- Tse JY, Wu S, Shinagare SA, Lauwers GY, Yilmaz O, Wu CL, Deshpande V. Peutz-Jeghers syndrome: a critical look at colonic Peutz-Jeghers polyps. Mod Pathol. 2013;26(9):1235–40
- Shepherd NA, Griggs RKL. Bowel cancer screening-generated diagnostic conundrum of the century: pseudoinvasion in sigmoid colonic polyps. Mod Pathol. 2015;28(Suppl 1):S88–S94
- Wagner A, Aretz S, Auranen A, Bruno MJ, Cavestro GM, Crosbie EJ, Goverde A, Jelsig AM, Latchford AR, van Leerdam ME, Lepisto AH, Puzzono M, Winship I, Zuber V, Möslein G. The Management of Peutz–Jeghers Syndrome: European Hereditary Tumour Group (EHTG) Guideline. J Clin Med. 2021;10(3):473
Presented by
Dr. Julia Azevedo, Porto, Portugal, and Dr. Cord Langner, Graz, Austria.