-
Die Universität
- Herzlich willkommen
- Das sind wir
- Medien & PR
-
Studium
- Allgemein
- Studienangebot
- Campusleben
-
Forschung
- Profil
- Infrastruktur
- Kooperationen
- Services
-
Karriere
- Arbeitgeberin Med Uni Graz
- Potenziale
- Arbeitsumfeld
- Offene Stellen
-
Diagnostik
- Patient*innen
- Zuweiser*innen
-
Gesundheitsthemen
- Gesundheitsinfrastruktur
Case of the Month
September 2025
Nodular anal lesion in a 54-year-old woman.
Diagnosis
Poorly differentiated, solid anal gland carcinoma, p16 positive.
Comment
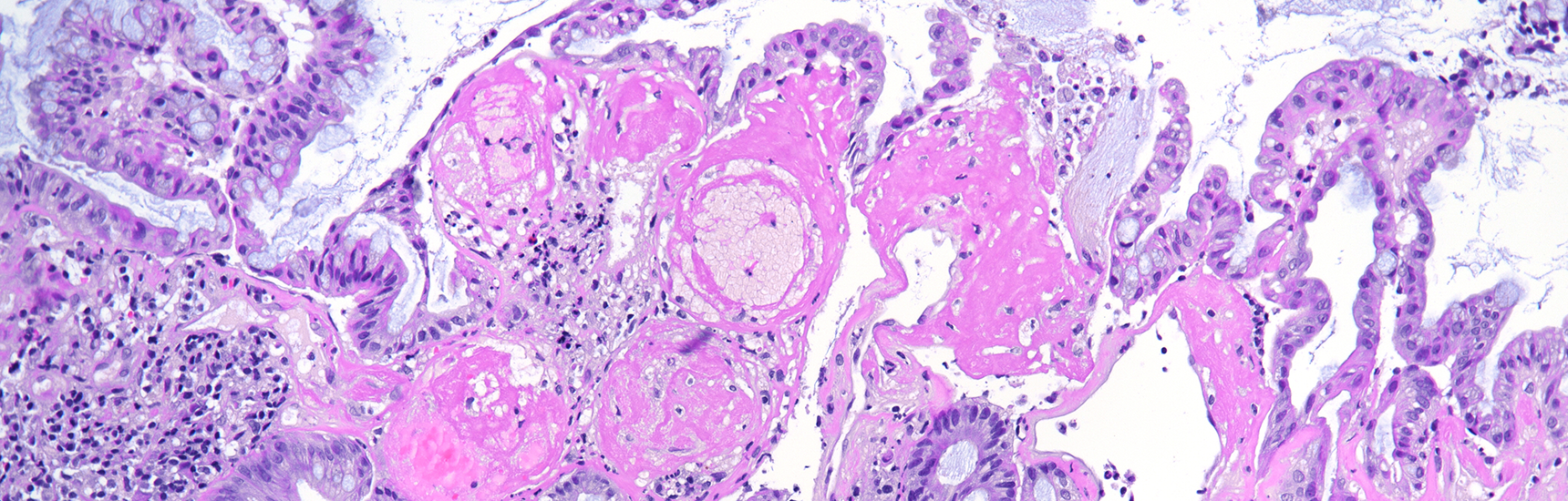
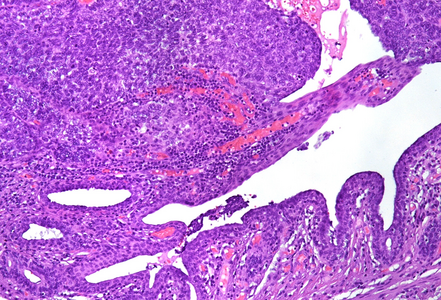
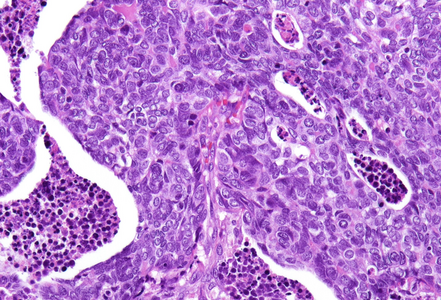
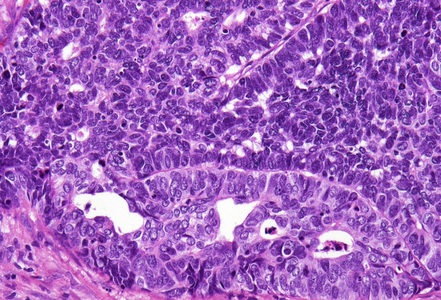
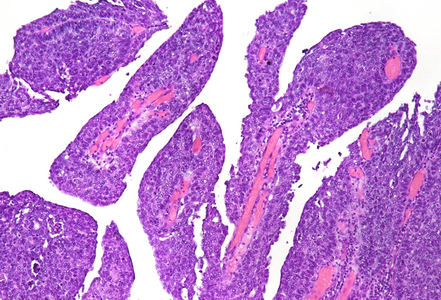
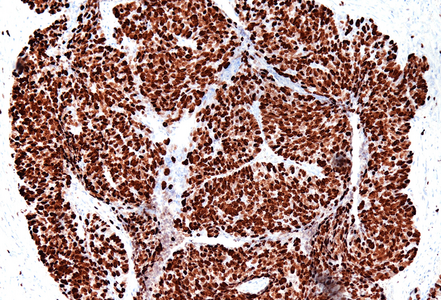
Histological examination showed a solid neoplasm with a predominantly nodular to lobulated growth pattern, frequently exhibiting peripheral palisading and central areas of comedonecrosis, located in the anal transitional zone (Panel A). The neoplastic cells were small to medium-sized, with scant cytoplasm, hyperchromatic nuclei, and inconspicuous nucleoli (Panel B). Glandular differentiation was minimal to absent (Panel C). The lesion showed focal connection with the overlying surface epithelium, which exhibited areas of papillary architecture with downward extension of neoplastic nests into the underlying stroma (Panel D). The overall morphological features were consistent with a poorly differentiated carcinoma, not clearly indicating glandular and/or squamous differentiation.
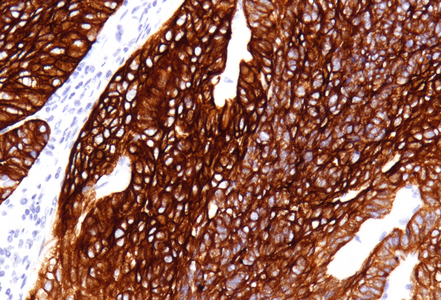
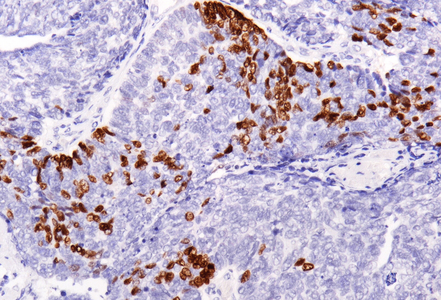
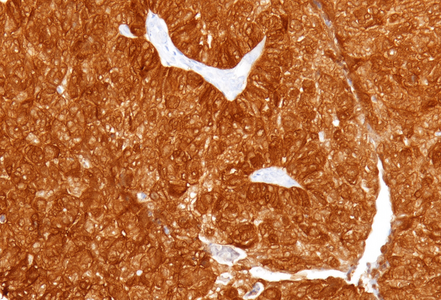
Immunohistochemistry revealed diffuse and strong cytoplasmic positivity for CK7 (Panel E) and CK19. Focal nuclear positivity was observed for p40 and p63 (Panel F). p16 immunostaining was diffusely positive, supporting a high-risk HPV-associated pathogenesis (Panel G). The tumor cells were negative for CK20, CK5/6, CDX2, synaptophysin, chromogranin, CD56, SOX10, PAX8, GATA3, TTF-1 and hormonal receptors. The Ki-67 proliferation index was markedly high (>90%) (Panel H). The immunohistochemical profile prompted a final diagnosis of poorly differentiated, presumably HVP-induced cancer originating from the ana glands, with a predominantly solid adenocarcinoma growth pattern yet minor squamous differentiation.
Anal adenocarcinoma is a rare malignancy that arises within the glandular epithelium of the anal canal. These tumors can be broadly categorized into mucosal and extramucosal types. Mucosal adenocarcinomas originate from the luminal mucosa and is of intestinal type, often resembling distal rectal adenocarcinomas. In contrast, extramucosal adenocarcinomas may develop from anal glands, pre-existing anal chronic fistula, or other structures (malformations or embryological remnants) and are further subtyped into anal gland adenocarcinoma, fistula-associated adenocarcinoma, and non–anal gland/non–fistula-associated adenocarcinoma.
Anal gland adenocarcinoma, with origin in the anal glands or ducts, is exceedingly rare. It typically presents as a painful perianal mass and is often clinically misinterpreted as benign disease such as hemorrhoids, contributing to delayed diagnosis. Most tumors are deeply seated in the perianal tissue, lack a luminal component, and may demonstrate pagetoid spread of carcinoma cells in the overlying epithelium. The histological spectrum of this subtype remains yet to be well defined. Typical cases exhibit haphazardly dispersed glands with cuboidal cells and scant mucin. Unusual subtypes may manifest solid growth or prominent mucin production.
Immunohistochemistry is crucial for diagnosis: anal gland-type tumors are typically CK7 and MUC5AC positive and CK20 and CDX2 negative, aiding in their distinction from rectal adenocarcinomas, which show exactly the opposite pattern. CK5/6 and p63, positive in benign anal glands, are usually negative in anal gland carcinomas. This immunoprofile also assists in distinguishing them from other differentials, such squamous cell carcinoma, anal mucoepidermoid carcinoma, neuroendocrine carcinomas and metastatic tumors from the gynecologic tract or breast.
HPV is implicated in a minority of anal adenocarcinomas, particularly those arising in the anal transitional zone. These cases often express p16 and may demonstrate better prognoses. However, overall, anal gland adenocarcinomas show a poorer prognosis than squamous cell carcinoma of the same region, with reported five-year survival rates ranging from 5–40%. Prognosis correlates strongly with TNM stage and histologic grade.
For further reading
- Balachandra B, Marcus V, Jass JR. Poorly differentiated tumours of the anal canal: a diagnostic strategy for the surgical pathologist. Histopathology. 2007;50(2):163–174
- Lisovsky M, Patel K, Cymes K, Chase D, Bhuiya T, Morgenstern N. Immunophenotypic characterization of anal gland carcinoma: loss of p63 and cytokeratin 5/6. Arch Pathol Lab Med. 2007;131(8):1304–1311
- Byrnes K, Liu X. Challenging and newly emerging neoplastic diseases in anal canal and their mimics. Hum Pathol Rep. 2022;28:300649
- Liao X. A review and update on epithelial tumors of the anal canal. J Clin Transl Pathol. 2022;2(4):149–158.
Presented by
Dr. Julia Azevedo, Porto, Portugal, and Dr. Cord Langner, Graz, Austria.